Published online Mar 27, 2022. doi: 10.4254/wjh.v14.i3.583
Peer-review started: August 1, 2021
First decision: September 29, 2021
Revised: October 31, 2021
Accepted: February 19, 2022
Article in press: February 19, 2022
Published online: March 27, 2022
Processing time: 234 Days and 19.7 Hours
Liver transplantation (LT) has become an acceptable curative method for children with several liver diseases, especially irreversible acute liver failure and chronic liver diseases. King Chulalongkorn Memorial Hospital is one of Thailand’s largest liver transplant centers and is responsible for many pediatric cases.
To report the experience with pediatric LT and evaluate outcomes of living-related vs deceased-donor grafts.
This evaluation included children who underwent LT between August 2004 and November 2019. Data were retrospectively reviewed, including demographics, diagnoses, laboratory values of donors and recipients, the pediatric end-stage liver disease (PELD) or model for end-stage liver disease (MELD) score, graft source, wait time, perioperative course, postoperative complications, and survival rates. Continuous data were reported using the median and interquartile range. The Mann–Whitney U-test was used to compare the wait time between the living-related and deceased-donor groups. The chi-square or Fisher's exact test were used to compare the frequencies of between-group complications. Survival rates were calculated using the Kaplan–Meier method.
Ninety-four operated pediatric liver transplant patients were identified (54% were females). The median age at transplantation was 1.2 (0.8-3.8) years. The median PELD and MELD scores were 20 (13-26.8) and 19.5 (15.8-26.3), respectively. Most grafts (81.9%) were obtained from living-related donors. The median wait time for the living donors was significantly shorter compared with the deceased donors at 1.6 (0.3-3.1) mo vs 11.2 (2.1-33.3) mo (P = 0.01). Most patients were diagnosed with biliary atresia (74.5%), and infection was the most common complication within 30 d post-transplantation (14.9%). Without a desensitization protocol, 9% of transplants were ABO-incompatible. Eight hepatitis B core antibodies (anti-HBc)-negative recipients received positive anti-HBc grafts without different observed complications. The overall survival rate was 93.6% and 90.3% at 1 and 5 years, respectively. No graft loss during follow-up was noted among survivors.
A significant number of pediatric LT cases were reported in Thailand. Based on relatively comparable outcomes, ABO-incompatible and HBc antibody-positive grafts may be considered in an organ shortage situation.
Core Tip: Pediatric liver transplantation (LT) is an acceptable life-saving operation for several chronic liver diseases and irreversible acute liver failure. This single-center data was analyzed from pediatric LTs performed between August 2004 and November 2019. This study evaluated the most extensive series of pediatric liver transplant recipients in Thailand in the past two decades. Preoperative and postoperative data, including complications and survival, were reviewed. The overall 5-year survival rate was > 90%. In addition, the satisfying outcomes of ABO-incompatible living-donor and hepatitis B core antibody-positive graft transplantation were also highlighted.
- Citation: Prachuapthunyachart S, Sintusek P, Tubjareon C, Chaijitraruch N, Sanpavat A, Phewplung T, Wanawongsawad P, Intrarakamhang AL, Chongsrisawat V. Pediatric liver transplantation outcomes from a single center in Thailand. World J Hepatol 2022; 14(3): 583-591
- URL: https://www.wjgnet.com/1948-5182/full/v14/i3/583.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i3.583
Liver transplantation (LT) has long been accepted as the standard treatment for end-stage liver disease due to acute fulminant hepatic failure and various chronic liver disorders. Starzl et al[1] were among the first group to demonstrate the possibility of this procedure in 1963 at the University of Colorado, Denver, CO, United States. This attempt faced several surgical and hemostatic challenges, requiring a very high dose of steroids and mercaptopurine due to the lack of effective immunosuppression regimens. The 1-year patient survival was about 30% at that time. However, the 1-year survival improved, close to 70%, because of the introduction of immunosuppressive drugs in the early 1980s[2]. Better organ-preservation techniques, enhanced surgical skills, and the availability of more effective immunosuppressive agents are also responsible for improved success rates. The shortage of organs from deceased donors led to a rapid expansion of living-donor programs, especially for children. The first successful living-donor LT was performed in 1989 in a pediatric patient[3]. The traditional indications for LT in children include end-stage liver disease with a predicted life expectancy of < 1 year, acute liver failure, unresectable hepatic tumors, and liver-based metabolic defects. The 5-year success rates for graft and patient survival for these life-threatening indications have been reported at a range of 85%-90%[4]. This success has led to an understandable urge to slightly contemplate more nontraditional indications for LT, including growth failure, intractable pruritus, or bone mass loss from cholestatic disorders, a neurodevelopmental abnormality from metabolic liver disease, and liver tumors in the absence of significant extrahepatic disease.
The first human LT was performed in Thailand at King Chulalongkorn Memorial Hospital, Bangkok, Thailand, on 28 November 1987 by Sriwatanawongsa et al[5]. Later, Nonthasoot et al[6] reported outcomes of LT from 1 January 2002 to 30 June 2013 for 120 adults and 24 pediatric LT cases. The data showed that the 1- and 5-year survival rates improved to 86% and 72%, respectively. As for pediatric patients, 16 and 8 had living-donor and cadaveric LT, respectively. The median age was 2 years old, and the most common primary indication was biliary atresia (83.3%). Patient survival at 1 and 5 years was 96% and 91%, respectively.
The objective of this study was to report the experience with pediatric LT performed at the center of the current study and evaluate outcomes of living-related vs deceased-donor grafts.
All pediatric liver transplant recipients who underwent LT at the center of this study between August 2004 and November 2019 were included. The data of all pediatric LT cases, including demographics, diagnoses, laboratory values of donors and recipients, the pediatric end-stage liver disease (PELD) or model for end-stage liver disease (MELD) score, graft source, wait time, perioperative course, immunosuppression type, postoperative complications, causes of death, and survival times, were retrospectively reviewed. The follow-up time was the duration in months from LT to the latest date of a doctor visit. Laboratory data were collected from preoperative assessment in both donors and recipients, including the ABO blood group and viral serology [hepatitis B surface antigen, hepatitis B surface antibody (anti-HBs), hepatitis B core antibody (anti-HBc), hepatitis C virus antibody, immunoglobulin G antibody to Epstein–Barr virus, and immunoglobulin G antibody to cytomegalovirus (anti-CMV IgG)]. Postoperative complications were categorized into three groups according to time to events after transplantation as early complications (within 30 d), middle complications (between 30 d and 1 year), and late complications (after 1 year). CMV viremia was defined as a detectable virus in blood quantified at ≥ 1000 DNA copies/mL using quantitative polymerase chain reaction or comparable positive antigenemia assay. Patient survival was defined as the time between LT and patient mortality. Moreover, graft survival was defined as the time between LT and graft loss, either by patient mortality or by graft failure necessitating retransplantation.
Statistical analyses were performed using SPSS 22.0 for Windows (SPSS Inc. Chicago, IL, United States). Categorical data were represented as numbers with percentages. Moreover, continuous data were reported using medians and interquartile ranges. The wait time was compared between the living-related and deceased-donor groups using the Mann-Whitney U-test. Frequencies of complications between groups were compared using chi-square or Fisher's exact test as appropriate. The survival rates were plotted using the Kaplan–Meier curves, and the differences in selected factors were evaluated using the log-rank test. Statistical significance was defined by P values < 0.05.
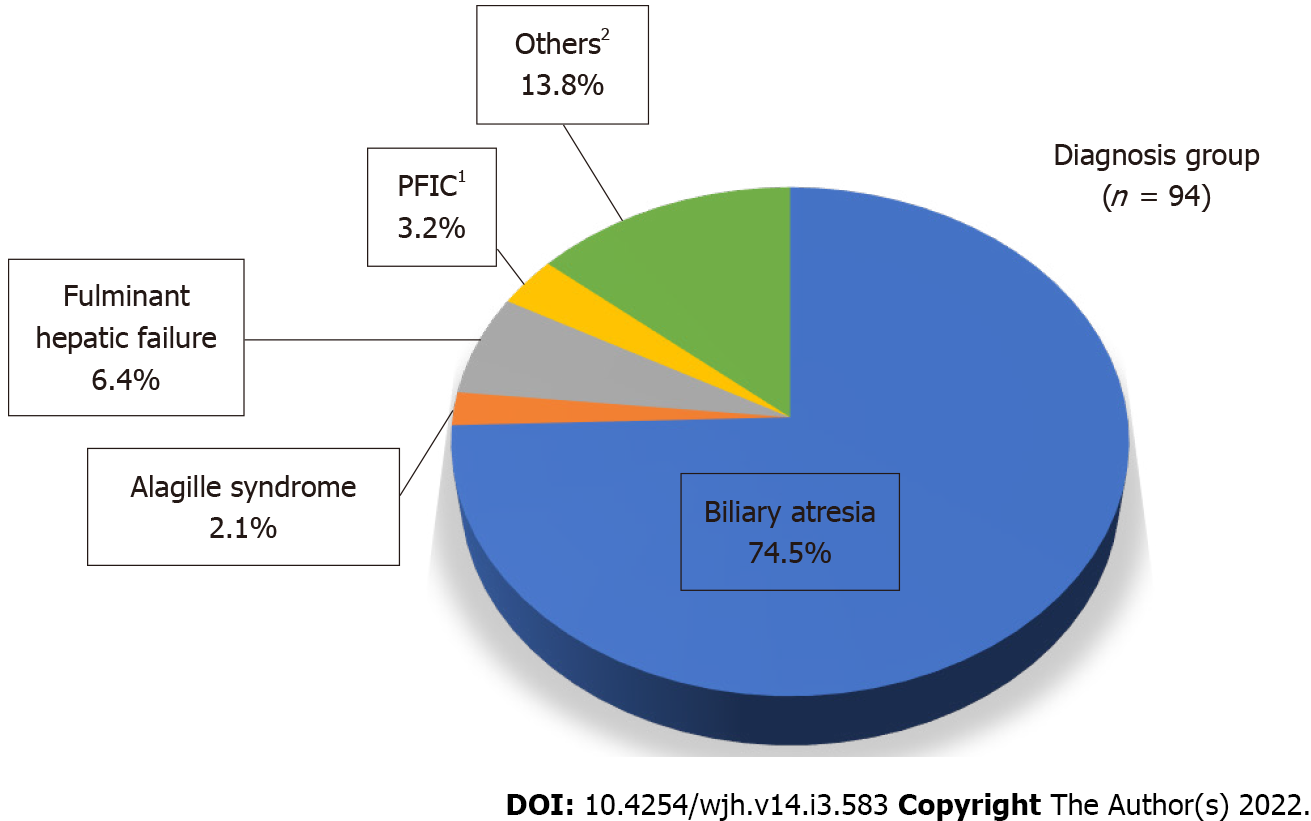
The most common diagnosis was biliary atresia (74.5%) in all 94 pediatric transplant recipients identified during the study period. Other less common diagnoses included fulminant hepatic failure, progressive familial intrahepatic cholestasis, Alagille syndrome, and others (Figure 1). Table 1 shows the patients’ characteristics. Most patients were < 2 years old (64.9%). The median age at transplantation was 1.2 (0.8–3.8) years, and significantly lower in the living-donor group [1.1 (0.8-1.9) years] compared with the deceased-donor group [9.7 (3.5-13.5) years; P < 0.001]. The median wait time for the living donors was significantly shorter than that for deceased donors at 1.6 (0.3–3.1) mo vs 11.2 (2.1–33.3) mo (P = 0.01). The median follow-up time was 4.0 (2.2–7.3) years.
| Data set | Living donors (n = 77) | Deceased donors (n = 17) | Total (n = 94) | |
| Sex | Male (n, %) | 36 (46.8) | 7 (41.2) | 43 (45.7) |
| Age (yr)1 | Median (IQR) | 1.1 (0.8-1.9) | 9.7 (3.5-13.5) | 1.2 (0.8-3.8) |
| Diagnosis of biliary atresia (n, %) | 59 (76.6) | 11 (64.7) | 70 (74.5) | |
| PELD score (age < 12 yr, n = 84) | Median (IQR) | 19 (12.5-26) | 25 (17.5-31.3) | 20 (13-26.8) |
| n | 74 | 10 | 84 | |
| MELD score (age ≥ 12 yr, n = 10) | Median (IQR) | 19 (18-19) | 23 (15-30) | 19.5 (15.8-26.3) |
| n | 3 | 7 | 10 | |
| Wait time (mo)2 | Median (IQR) | 1.6 (0.3–3.1) | 11.2 (2.1–33.3) | 1.7 (0.4–4.0) |
Table 2 shows the serology data. Eight ABO-incompatible transplants were performed in the living-donor group in infants (< 1 year old) without a desensitization protocol. Positive donor anti-HBc grafts were transplanted to eight patients with negative anti-HBc (8.5%). Five of the 54 recipients were anti-HBc-positive, although every patient in this group received negative donor anti-HBc grafts. All recipients received at least one primary hepatitis B Virus (HBV) vaccination at birth and a booster dose before LT. HBc antibody-positive graft recipients also received lamivudine prophylaxis for de novo HBV infection after LT. Most recipients (74/84, 88.1%) had positive anti-CMV IgG. All CMV-naïve recipients (10/84, 11.9%) received positive anti-CMV IgG grafts.
| Data set | Living donors (n = 77) | Deceased donors (n = 17) | Total (n = 94) | |
| ABO incompatibility1 | n, % | 8 (10.4) | 0 (0) | 8 (8.5) |
| Positive donor anti-HBc | n, % | 4 (5.2) | 4 (23.5) | 8 (8.5) |
| Anti-CMV IgG (n, %)2 | D+/R- | 8 (11.0) | 2 (18.2) | 10 (11.9) |
| D+/R+ | 62 (84.9) | 9 (81.8) | 71 (84.5) | |
| D-/R+ | 3 (4.1) | 0 (0) | 3 (3.6) | |
| D-/R- | 0 (0) | 0 (0) | 0 (0) | |
| Recipient positive serology (n/total, %) | HBsAg | 0/44 (0) | 0/9 (0) | 0/53 (0) |
| Anti-HBs | 33/43 (76.7) | 3/10 (30) | 36/53 (67.9) | |
| Anti-HBc | 3/44 (6.8) | 2/10 (20) | 5/54 (9.3) | |
| Anti-HCV | 1/42 (2.4) | 0/10 (0) | 1/52 (1.9) | |
| Anti-EBV IgG | 31/43 (72.1) | 8/9 (88.9) | 39/52 (75) | |
All patients postoperatively received corticosteroids in conjunction with at least one main T-cell suppression immunosuppressant. Tacrolimus (78.7%) was the preferred calcineurin inhibitor over cyclosporine (21.3%). Other additional immunosuppressive drugs used included azathioprine (30.9%), mycophenolate mofetil (26.6%), and sirolimus (3.2%). Apart from corticosteroids, more than half of the patients (58.5%) required a combination of two or more immunosuppressive agents.
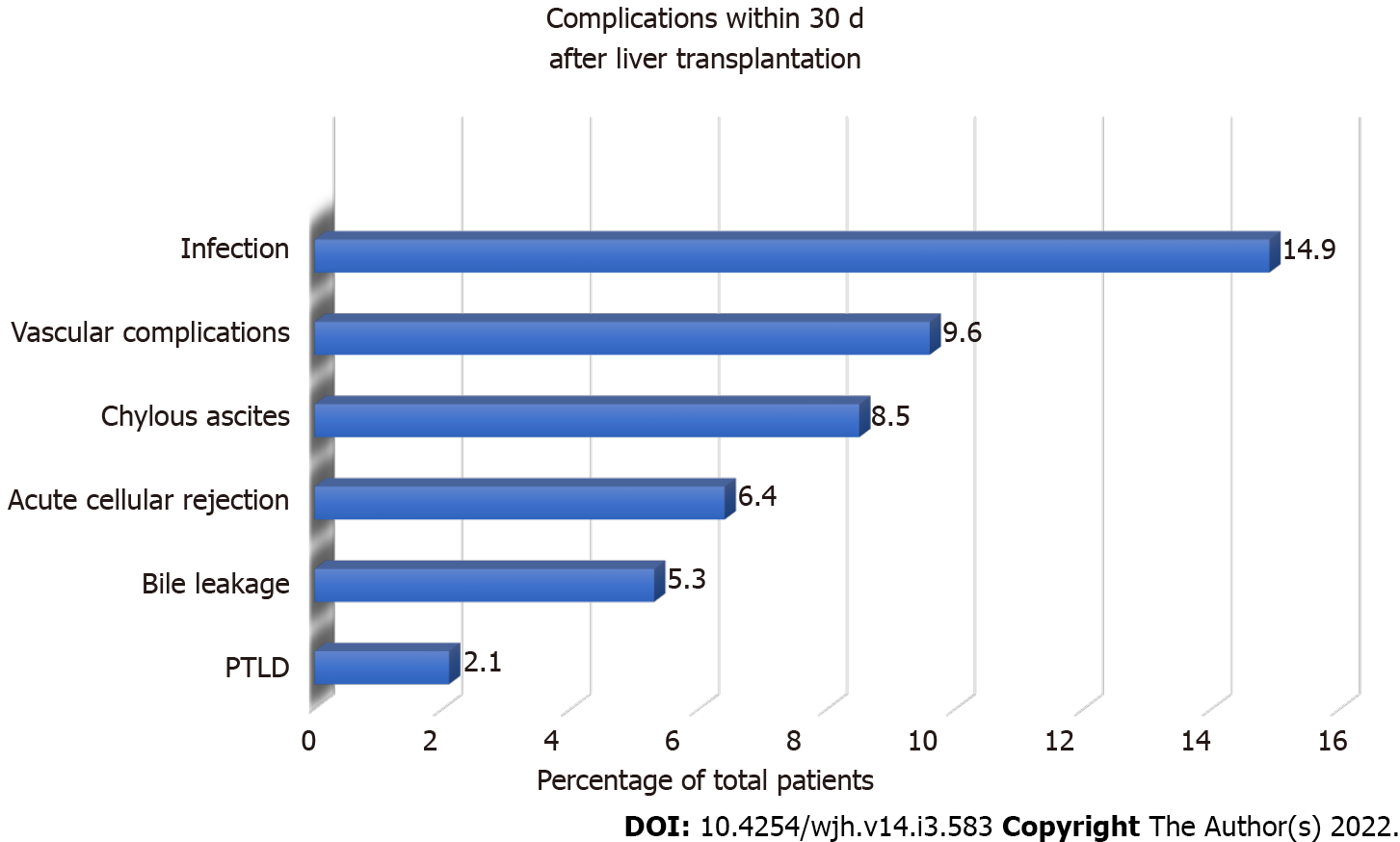
Infection was the most common early complication (within 30 d after LT; 14.9%). Other less common early complications included vascular complications, chylous ascites, acute cellular rejection, bile leakage, and post-transplant lymphoproliferative disorder (PTLD, Figure 2). One patient who received living-related-donor LT had hepatic artery thrombosis, which required a second emergent deceased-donor LT. PTLD occurred in 10.6% of the patients within the first year after transplantation, whereas 3.2% developed PTLD after the first year. Early CMV viremia within the first year after LT was detected in 40% of CMV-naïve patients compared with 4.8% of CMV-seropositive patients (P = 0.004). Food allergy was found in 23 patients (24.5%), 65.2% of which were de novo food allergies, defined as the occurrence of allergic symptoms after LT. No donors with a history of allergy were identified among these de novo food allergy recipients. The median age at LT was 13 (10-19.8) mo, whereas the median time to the event was 148 (92-347) d. The common culprits were cow milk (66.7%), egg (46.7%), and wheat (20%).
No observed different vascular, infection, or rejection complications were noted in the ABO-incompatible and positive donor anti-HBc graft transplantation cases. Four patients among the eight ABO-incompatible LT recipients had no complications. One patient developed early sepsis, candida urinary tract infection, hepatic artery stenosis, portal vein thrombosis, and bile leakage. Two patients developed PTLD within the first year after LT. One patient expired 4 d after LT due to sepsis.
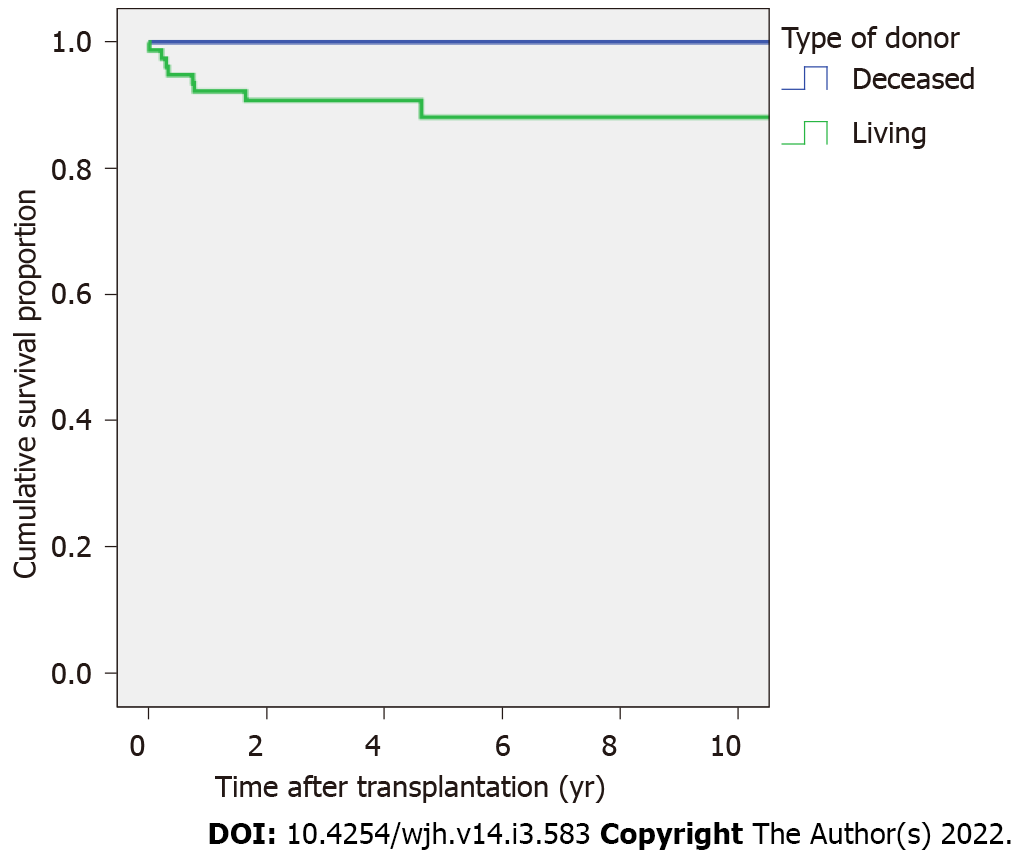
The overall survival rate was 93.6% and 90.3% at 1 and 5 years (living-donor group = 92.2% and 88.1% at 1 and at 5 years, respectively; deceased-donor group = 100% at 1 and 5 years), respectively (Figure 3). All patients with acute liver failure (6.4%) survived after LT with normal neurodevelopmental outcomes. Six (75%) of the eight total deaths occurred within the first year after transplantation. The preoperative diagnoses were biliary atresia (7/8, 87.5%) and bile acid synthesis disorder (1/8, 12.5%). The causes of mortality were sepsis (three patients), acute renal failure and shock (two patients), bacterial pneumonia (one patient), and unknown (two patients).
Pediatric LT is considered the standard management for children with several chronic liver diseases and irreversible acute liver failure. King Chulalongkorn Memorial Hospital, affiliated with the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, is the leading hospital for pediatric LT and is responsible for most procedures in Thailand. At the aforementioned hospital, 23 children underwent LT (average, 2.6 cases per year) from 2004 to 2013. The remaining 71 patients underwent LT from 2014 to November 2019 (average, 14.2 cases per year). The most common indication for pediatric LT in the current series was biliary atresia (74.5%), consistent with other series previously reported[7-9]. The liver graft allocation policy for pediatric recipients in the current study includes the use of living-donor grafts as default and deceased-donor grafts when the former is unavailable or impossible due to donor limitations. Most grafts came from living donors (81.9%); however, this rate was lower than recent reports from Japan and Turkey. Kasahara et al[7] reported on 414 and 22 Living- and deceased-donor transplantations up to 2016 from the Organ Transplantation Center at the National Center for Child Health and Development in Japan. In Turkey, Yankol et al[8] reported on 135 cases of pediatric LT, 91.4% of which involved living donors. However, the rate of living-donor LT in the current study was higher than in Western and Middle Eastern countries, including centers in the United States (6%)[9] and Iran (40.6%)[10]. Regarding other patient characteristics, the median age of patients [1.2 (0.8-3.8) years] in the current study is relatively lower compared with other studies. Moreover, the median PELD and MELD scores were 20 (13-26.8) and 19.5 (15.8-26.3), respectively, which is comparable to other studies[8,9].
The most common early complication in the current series was an infection, consistent with other reports. Nikeghbalian et al[10] reported in-hospital complications encountered by 34.7% of the patients, and the most common being infections (26.8%), bleeding (23.4%), and vascular complications (18%). The United States Studies in Pediatric Liver Transplantation (SPLIT) group[11] also reported that infection was the most common complication. It occurred in nearly half of all patients (46%) and could be as severe as multisystem organ failure or cardiopulmonary failure. Infants, which accounted for most of the pediatric LT cohorts in the current study, were at the highest risk of developing an infection. Eight pediatric recipients received positive anti-HBc grafts and were started on lamivudine after LT. These patients had good graft survival and complications that were comparable to the negative anti-HBc group without HBV infection reactivation that is likely because of the antiviral prophylaxis. In addition, anti-HBs and revaccination of patients with low or undetectable anti-HBs before LT were also monitored because keeping the anti-HBs > 200 mIU/mL before LT could be sufficient to prevent de novo HBV infection[12]. However, one case with biliary atresia and cirrhosis diagnosis developed de novo HBV infection after receiving a negative anti-HBc liver graft. This patient had a high anti-HB titer (anti-HBs > 1000 IU/L) before LT but had elevated transaminases at 3.83 years after LT before the HBV infection was diagnosed. Sintusek et al[13] reported the unexpectedly high prevalence of HBV immunity loss after LT (46%, 57%, and 82% at 1, 2, and > 3 years following LT). Positive anti-HBc grafts may be considered with relatively positive outcomes in the face of organ shortages for LT. Oral antivirals for HBV and careful monitoring of viral serology should be performed in endemic areas given the high prevalence of HBV immunity loss after LT.
Regarding vascular complications, hepatic artery thrombosis after pediatric LT ranges from 5.7% to 8.4% and is an important cause of graft loss[11]. Portal vein thrombosis is another vascular complication that can occur after LT. Vascular complications occurred in 9.6% of the cohorts of the current study. One patient, who underwent LT in 2010, developed hepatic artery thrombosis and required a second LT. However, the rate of vascular complications significantly declined with careful selection of donors and ultrasound monitoring. Doppler ultrasound after reperfusion was routinely performed to ensure good vascular flow, and daily monitoring continued 1 wk after LT.
Herein, the good outcome of ABO-incompatible LT was highlighted in eight < 1-year-old pediatric recipients without a desensitization protocol. No differences in terms of complications or graft and patient survival were found. This is likely because differentiation and maturity of the immune system are closely tied to children’s age. Isoagglutinin titers in < 1-year-old infants are lower than adult levels[14]. In a systematic review and meta-analysis, Lee et al[15] reported comparable patient survival in both groups. However, the ABO-incompatible group was inferior regarding graft survival and several complications. Graft survival could be comparable in pediatric patients and those using rituximab. However, another more recent systematic review and meta-analysis by Kang et al[16] showed consistently lower patient and graft survival with pediatric ABO-incompatible LT than the ABO-compatible group. The authors concluded that ABO-incompatible LT is an important choice to consider for emergency LT in the absence of blood type-matching liver source although it is not an optimal treatment in terms of graft and patient survival rates.
Patient survival rates at 1 and 5 years after a pediatric LT are 97.3% and 94.2%, respectively, according to a recent SPLIT registry database from 2011 to 2018[17]. The survival rates from the largest single-center report on pediatric LT in Iran were up to 84.4% and 77.8% at 1 and 5 years, respectively[10]. Thus, the overall survival rates of the current series of 93.6% and 90.3% at 1 and 5 years, respectively, are comparable.
The limitations of the current study include the inherent nature of retrospective studies. Some detailed information may be missing from the medical records, including specific causes of mortality or mention of minor infections that could have occurred at home or other hospitals. Also, the pretransplant viral serology of donors and recipients was not available in all cases due to missing data from the records. The main strength of the current study is that it is the most extensive report on pediatric LT in Thailand covering the two decades. The experiences in the current study also highlighted a good proportion of living-donor LT in children with relatively comparable outcomes and excellent graft and patient survival compared to global data.
In conclusion, pediatric LT has been accepted as a life-saving procedure for patients with acute liver failure and chronic end-stage liver diseases. The transplant center of the current study is responsible for a large number of pediatric LT procedures in Thailand. Herein, the experiences in pediatric LT with excellent outcomes concerning survival and complications are reported. The current series was mostly comprised of living-related donor liver grafts, which had the advantage of shorter wait times compared to deceased-donor grafts. The experiences with ABO-incompatible LT in < 1-year-old patients without a desensitization protocol and HBc antibody-positive LT with relatively comparable outcomes were also highlighted, leading to the assertion that such grafts should be considered in the face of organ shortages. Overall, the results in the previous 15 years regarding pediatric LT are promising.
Pediatric LT has been accepted as a curative method for children with several liver diseases. The success rates have improved due to better organ-preservation techniques, enhanced surgical skills, and the availability of newer immunosuppressive agents. Organ shortage has become a rising problem worldwide, especially in Eastern countries.
King Chulalongkorn Memorial Hospital is the leading hospital in Thailand for pediatric LT. Several reports on pediatric LT were noted in the United States, Europe, Middle East, and East Asian countries. However, data from South East Asia, especially related to ABO-incompatible LT, are scarce.
The current study aimed to report experiences with pediatric LT performed at the center of this study and evaluate outcomes of living-related vs deceased-donor grafts.
The current retrospective study included 94 children who underwent LT and were followed up for a median time of 4 years thereafter. Data of donors and recipients, including postoperative complications and survival rates, were reviewed and analyzed.
In the current study, 94 pediatric LT performed at the center of this study were reported. The median age at transplantation was 1.2 (0.8-3.8) years. Most grafts (81.9%) were obtained from living-related donors. The median wait time for the living donors was significantly shorter than that for deceased donors at 1.6 (0.3-3.1) vs 11.2 (2.1-33.3) months (P = 0.01). Most patients were diagnosed with biliary atresia (74.5%), and infection was the most common complication within 30 d post-transplantation (14.9%). In addition, 9% of transplants were ABO-incompatible without a desensitization protocol. No observed different vascular, infection, or rejection complications were noted. Eight (8.5%) recipients who tested negative for HBc antibodies received positive anti-HBc grafts with no observed different infection or rejection complications. The overall survival rate was 93.6% and 90.3% at 1 and 5 years, respectively. No graft loss during follow-up was noted among the survivors.
Living-donor-related LT has saved many lives with shorter wait times compared with deceased-donor surgeries. Based on relatively comparable outcomes, ABO-incompatible and HBc antibody-positive liver grafts may be considered in the face of organ shortages. The survival results in the previous 15 years are promising.
The current study suggests that living-donor liver transplantation (LT) can save many lives and has a good outcome with shorter wait times in the face of organ shortage. ABO-incompatible LT can be considered in pediatric < 1-year-old recipients without a sensitization protocol. Hepatitis B core (HBc) antibody-positive liver grafts may also be used. Nonetheless, special attention should be focused on high titers of anti-hepatitis B surface before LT and lifelong postoperative antiviral prophylaxis. More studies on living-donor pediatric LT and protocols for these special donor groups are needed.
The authors would like to thank Dr. Pongrat Sirichindakul, the head of the Excellence Center of Organ Transplantation, its members, and all the transplant surgeons for their tremendous support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yagi H S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659-676. [PubMed] |
| 2. | Starzl TE, Klintmalm GB, Porter KA, Iwatsuki S, Schröter GP. Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981;305:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 374] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet. 1989;2:497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 414] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Kohli R, Cortes M, Heaton ND, Dhawan A. Liver transplantation in children: state of the art and future perspectives. Arch Dis Child. 2018;103:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Sriwatanawongsa V, Sangsubhan C, Dhitavat V, Tanprayoon T, Sriussadaporn S, Borirakchanyawat V, Suwangool P, Kyokong O, Niruthisard S, Vajarapongse K, Minaphinant K, Nivatvong S, Auwattanamongkol S, Puttisri A, Rajatapiti B. The first human orthotopic liver transplant in Thailand: A case report. Chula Med J. 1988;32:463-468. |
| 6. | Nonthasoot B, Sirichindakul B, Suphapol J, Taesombat W, Sutherasan M, Nivatvongs S. Orthotopic liver transplantation at King Chulalongkorn Memorial Hospital: a report. J Med Assoc Thai. 2015;98 Suppl 1:S127-S130. [PubMed] |
| 7. | Kasahara M, Sakamoto S, Fukuda A. Pediatric living-donor liver transplantation. Semin Pediatr Surg. 2017;26:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Yankol Y, Mecit N, Kanmaz T, Cimsit B, Cakaloglu Y, Acarli K, Kalayoglu M. Lessons Learned From Review of a Single Center Experience With 500 Consecutive Liver Transplants in a Region With Insufficient Deceased-Donor Support. Exp Clin Transplant. 2016;14:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Venick RS, Farmer DG, Soto JR, Vargas J, Yersiz H, Kaldas FM, Agopian VG, Hiatt JR, McDiarmid SV, Busuttil RW. One Thousand Pediatric Liver Transplants During Thirty Years: Lessons Learned. J Am Coll Surg. 2018;226:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Nikeghbalian S, Malekhosseini SA, Kazemi K, Arasteh P, Eghlimi H, Shamsaeefar A, Nikoupour H, Gholami S, Dehghani M, Dehghani SM, Bahador A, Salahi H. The Largest Single Center Report on Pediatric Liver Transplantation: Experiences and Lessons Learned. Ann Surg. 2021;273:e70-e72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, Song C; SPLIT Research Group. Impact of graft type on outcome in pediatric liver transplantation: a report From Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg. 2007;246:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, Wu YM, Chang MH, Lee PH. High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr. 2009;48:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Sintusek P, Posuwan N, Wanawongsawad P, Jitraruch S, Poovorawan Y, Chongsrisawat V. High prevalence of hepatitis B-antibody loss and a case report of de novo hepatitis B virus infection in a child after living-donor liver transplantation. World J Gastroenterol. 2018;24:752-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Egawa H, Oike F, Buhler L, Shapiro AM, Minamiguchi S, Haga H, Uryuhara K, Kiuchi T, Kaihara S, Tanaka K. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004;77:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Lee EC, Kim SH, Park SJ. Outcomes after liver transplantation in accordance with ABO compatibility: A systematic review and meta-analysis. World J Gastroenterol. 2017;23:6516-6533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Kang ZY, Liu W, Li DH. Comparison of clinical outcomes between ABO-incompatible and ABO-compatible pediatric liver transplantation: a systematic literature review and meta-analysis. Pediatr Surg Int. 2020;36:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Elisofon SA, Magee JC, Ng VL, Horslen SP, Fioravanti V, Economides J, Erinjeri J, Anand R, Mazariegos GV; Society of Pediatric Liver Transplantation Research Group. Society of pediatric liver transplantation: Current registry status 2011-2018. Pediatr Transplant. 2020;24:e13605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |